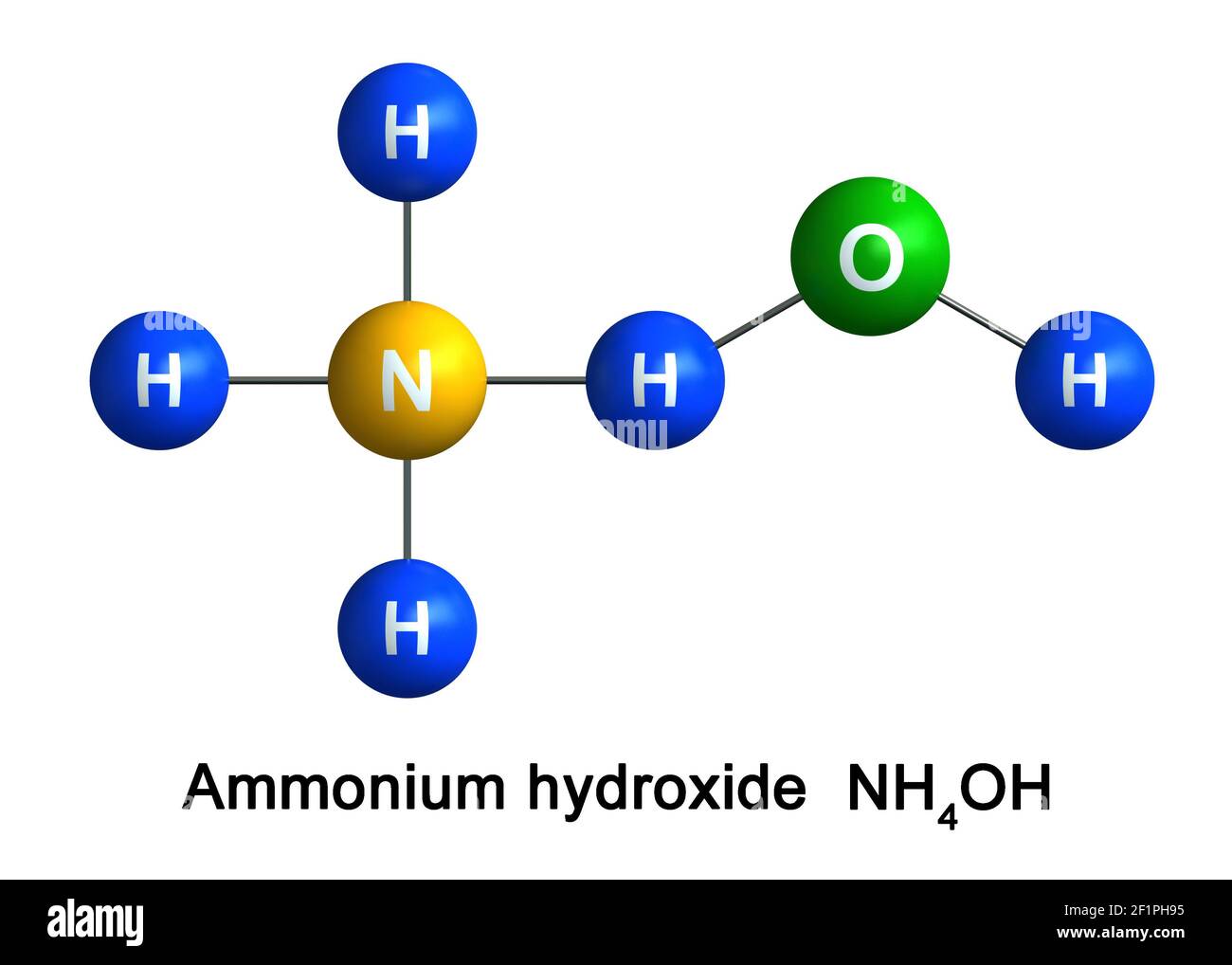
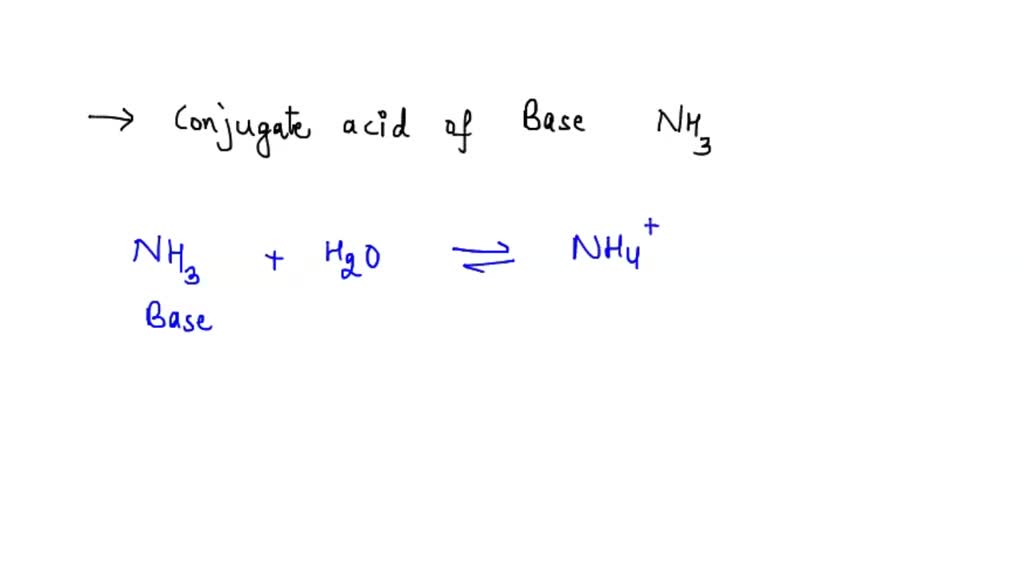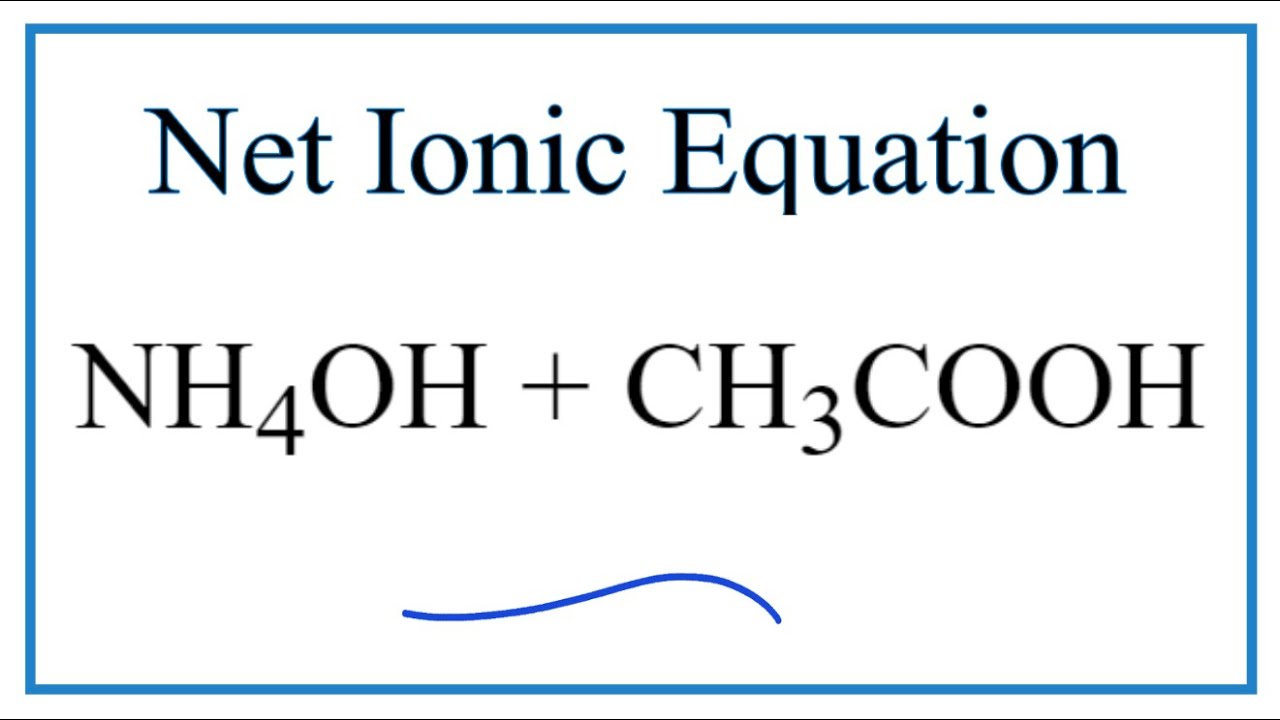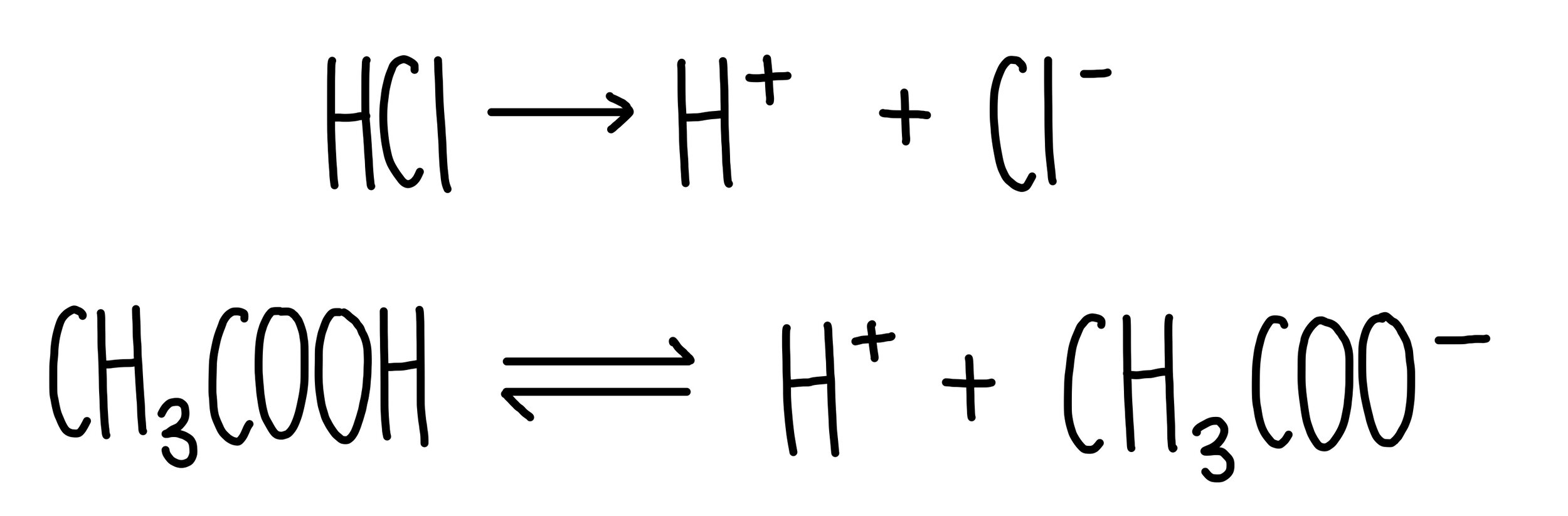SOLVED: NHANOz is the conjugate acid of the weak base ammonium hydroxide (NHAOH) and the strong acid nitric acid (HNO3). Kb for ammonium hydroxide is 1.8 x 10-5 according to the following

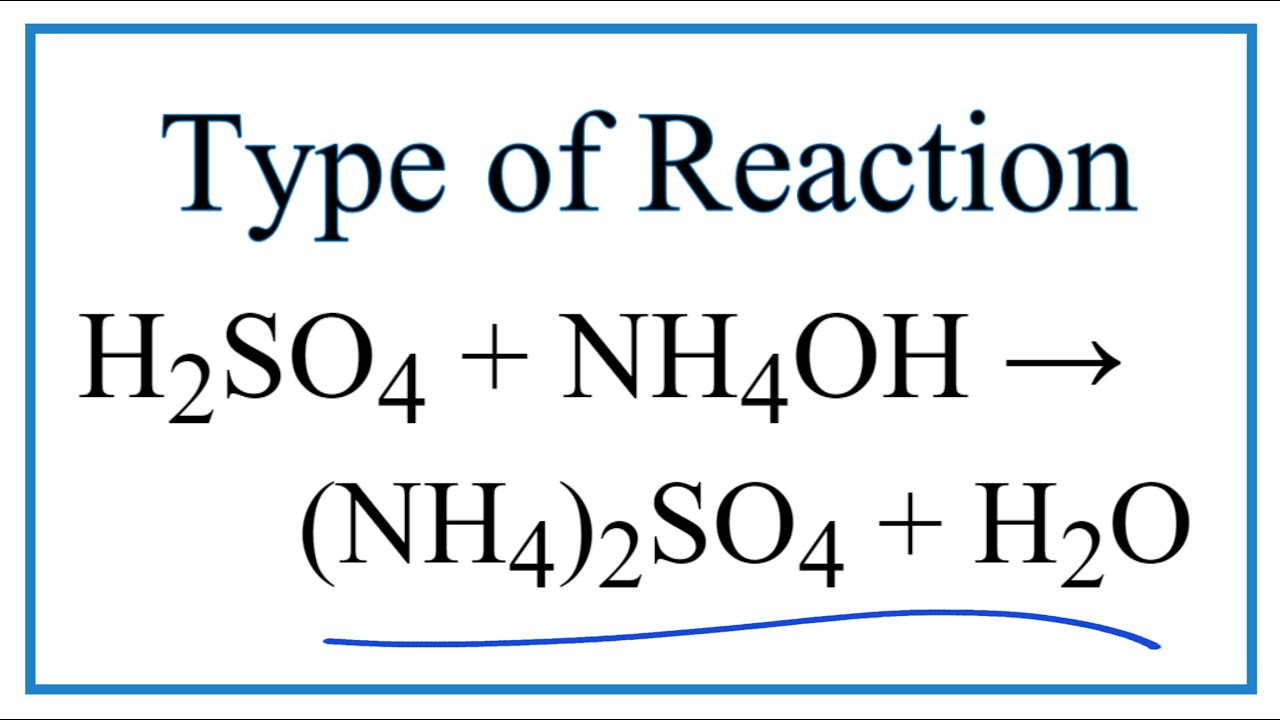
The enthalpy of neutralisation of NH4OH with HCl is - 51.46kJ/ mol^-1 and the enthalpy of neutralisation of NaOH with HCl is - 55.90kJ/ mol^-1 .The enthalpy of ionisation of NH4OH is:
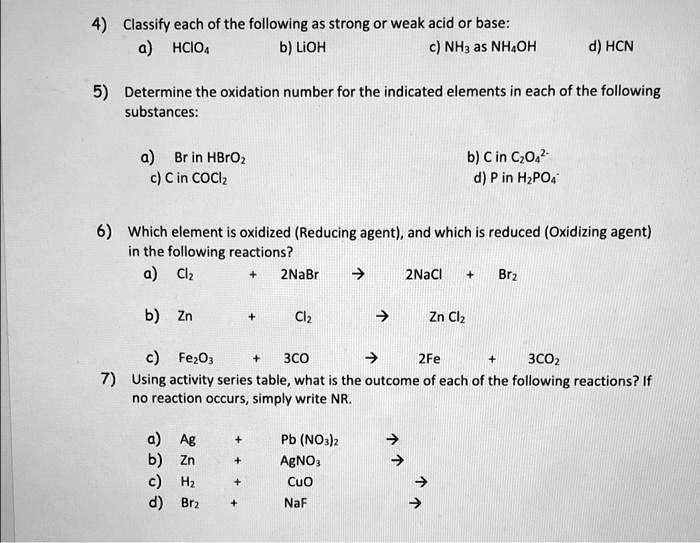
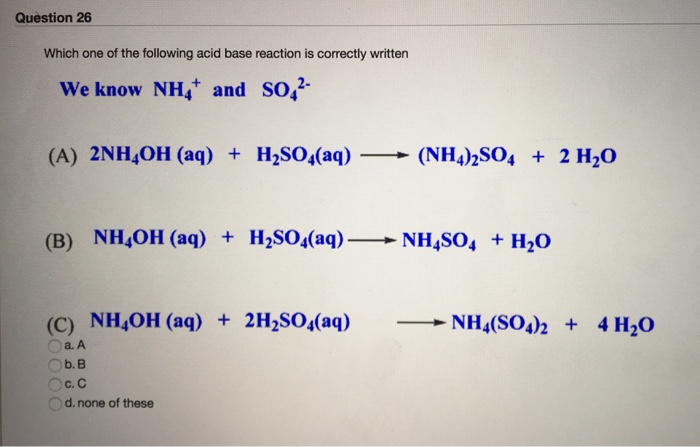
SOLVED: can someone please help with these questions? thanks! 4Classify each of the following as strong or weak acid or base: a)HCIO4 b)LiOH cNHas NH4OH d)HCN 5) Determine the oxidation number for
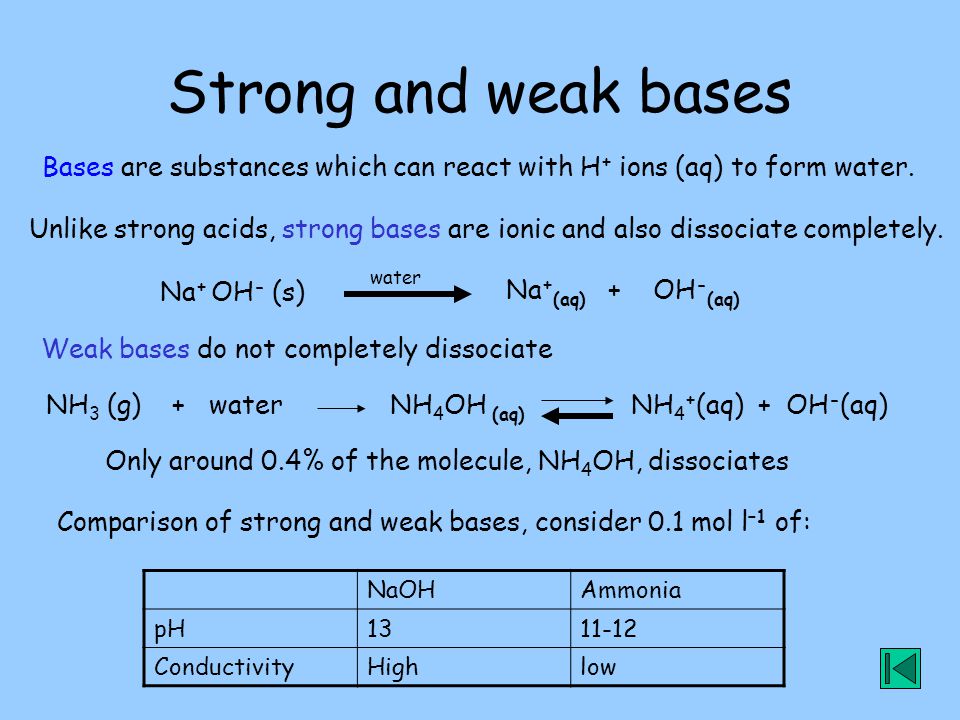
Chemical Equilibrium Equilibrium, strong and weak acids and bases, solutions, concentration and pH. - ppt video online download